
The DFW Hospital Council is posting blogs submitted by our Associate Members. The following was provided by Gerber. For guidelines, please contact Chris Wilson at chrisw@dfwhc.org.
Among infants, atopic dermatitis (AD) is the most common allergic disease. In fact, up to 1 in 5 infants will develop this skin condition by age 6 months of age, and it can persist into later childhood. An estimated 7.4 million yearly doctor visits occur in the U.S. for children with AD, with costs up to $3.8 billion annually.
Breastfeeding is the best way to feed a child. Multiple studies have shown the health benefits of breastfeeding, some of which include appropriate weight gain, lower risk of infections and even a reduced risk of AD. However, for healthy infants with a family history of allergy who are not exclusively breastfed, feeding certain hydrolyzed infant formulas during the first four months of life may help reduce the risk of developing AD compared to feeding formulas made with intact cow’s milk proteins. Because hydrolyzed formulas are milk based, they are not appropriate for infants who have a suspected or confirmed allergy to cow’s milk.
The relationship between reduced risk of developing atopic dermatitis and feeding 100% whey protein partially hydrolyzed infant formula (PHF-W), like Gerber® Good Start® Gentle formula, has been shown in several studies, but perhaps the most important is the German Infant Nutritional Intervention study (GINI). More than 2,000 infants were enrolled in this large independent study, and they have now been followed up to 15 years of age. At each time point reported, the PHF-W group showed significantly lower risk of developing AD compared with those who received intact cow’s milk protein-based formula (CMF).
Multiple trials in different patient cohorts have also shown results for AD risk reduction; though not all reached statistical significance, each showed a reduced risk of AD with PHF-W versus intact protein CMF. This has led to various academic societies, institutions and guidelines discussing their use in infants with a family history of allergy. In addition to reducing the risk of AD for high-risk infants (infants with a family history of allergy), there are cost-saving implications for the use of feeding PHF-W like Gerber® Good Start® Gentle formula in place of CMF for non-exclusively breast fed infants.
A health economics model was developed in 2016 that included the cost of formula, the incidence of AD, results from a physician survey about how atopic dermatitis is typically managed by U.S. pediatricians, and information from Nielsen data for specialty formula costs and from pharmaceutical sources for costs of creams and other medications physicians might prescribe for AD. Based on this model, the overall total net cost was $495 less for high-risk children initially fed PHF-W compared with CMF during the first six years of life, regardless of whether or not AD develops.
Allergic conditions are among the most common medical conditions affecting children in the U.S., and according to the CDC the prevalence of food and skin allergies increased in children under age 18 from 1997–2011. Not only does AD have high financial burden, it can also have an emotional one with significant impact on the quality of life of children and their caretakers due to scratching, pain, and sleep difficulties (which may result in daytime sleepiness), disruptions in play, and school and conduct problems.
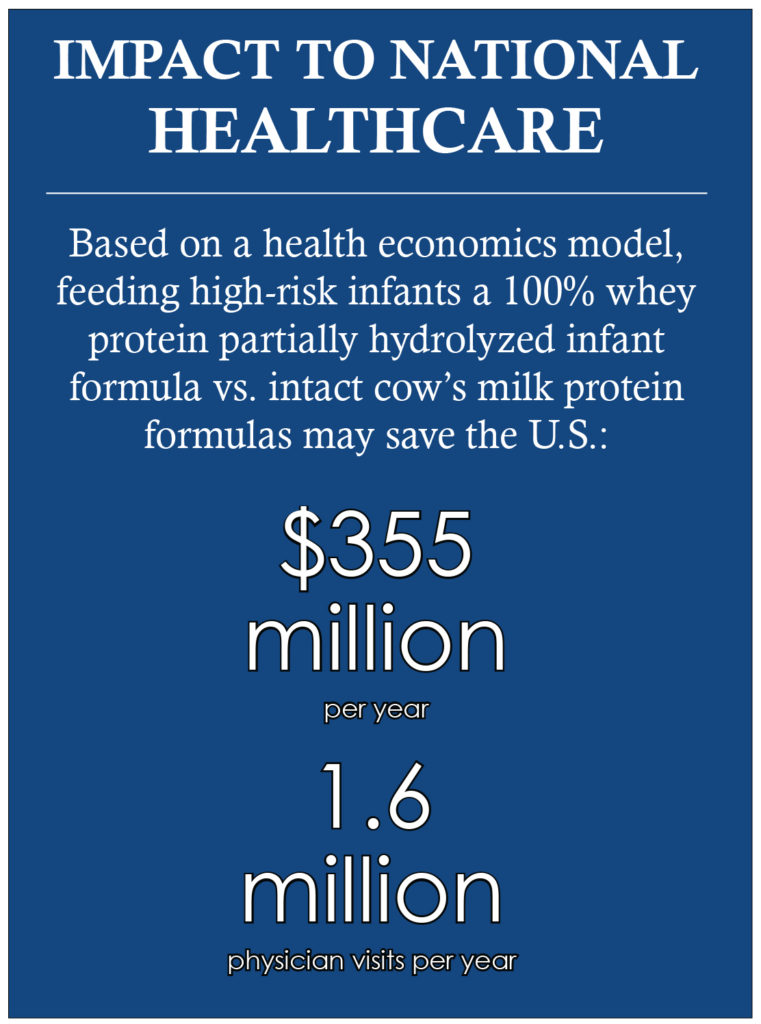
The use of PHF-W in infants with a family history of allergy has been found to be cost-effective and/or cost-saving compared with feeding CMF in several developed countries. The national impact of feeding PHF-W instead of CMF to all at risk U.S. formula fed infants who are not exclusively breastfed could provide savings of $355 million per year, and a total estimated 1.6 million avoided AD physician visits.
Therefore, the use of PHF-W instead of CMF in healthy at risk infants who are not exclusively breastfed may be expected to help reduce the risk of developing of AD, resulting in a net cost savings to the healthcare system and society.
RESOURCES
1. Jackson KD, et al. NCHS Data Brief 2013;121:1-8.
2. Moore MM, et al. Pediatrics 2004:113; 468-474.
3. Horii K, et al. Pediatrics 2007; 120:e527-34.
4. Mancini A, et al. Ped Derm 2007; 25:1-6.
5. von Berg A, et al. J Allergy Clin Immunol. Allergy Clin Immunol. 2003;111: 533–540.
6. von Berg A, et al. J Allergy Clin Immunol. J Allergy Clin Immunol 2007;119:718-25.
7. von Berg A, et al. J Allergy Clin Immunol. 2008; 121:1442–1447.
8. von Berg A, et al. J Allergy Clin Immunol. 2013;131:1565-73.
9. von Berg A, et al. Allergy 2015; DOI: 10.1111/all.12790.
10. Marini A, et al. Acta Paediatr Suppl 1996;414:1-21.
11. Chan YH, et al. J Paediatr Child Health 2002;38:84-8.
12. Vandenplas Y, et al. Eur J Pediatr 1995;154:488-94.
13. Greer F, et al. Pediatrics 2008;121:183-191.Boyce 2010
14. Fleischer D. J Allergy Clin Immunol: In Practice 2013;1:29-36.
15. Boyce JA, et al. NIAID 2010. NIH Publication No. 11-7700.
16. Bhanegaonkar A, et al. J Pediatr 2015; 166:1145-51.
17. Filanovsky MG, et al. J Pediatr 2016;169:284-90.
Gerber – Infant Feeding, reducing the risk of Atopic Dermatitis
06/20/2017